Life is too short for poor batch implementations. Many organizations are purchasing equipment—especially bioreactors—with an inappropriate batch philosophy, resulting in
Turning Biotech GMP Issues into Solutions...
Getting it right means blending deep expertise with tight process control, clean interfaces, and rock-solid regulatory compliance data.
The best way to show our range of services is through our project work postings below.
For ease, it’s grouped by our four areas of expertise.
Automation
Follow Us
Industrial Networking - IT/OT
Data Integrity
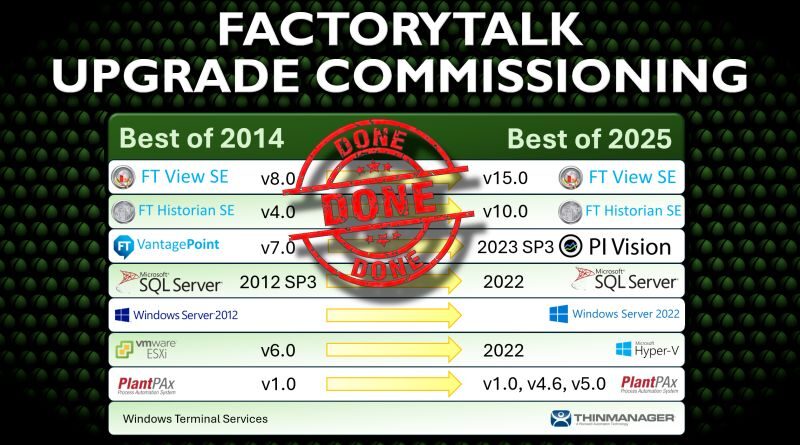
FactoryTalk Upgrade – Best of 2014 to Best of 2025
Finally got the go-ahead to upgrade a system I installed back in 2014, and the results are sweet. Zero downtime, more